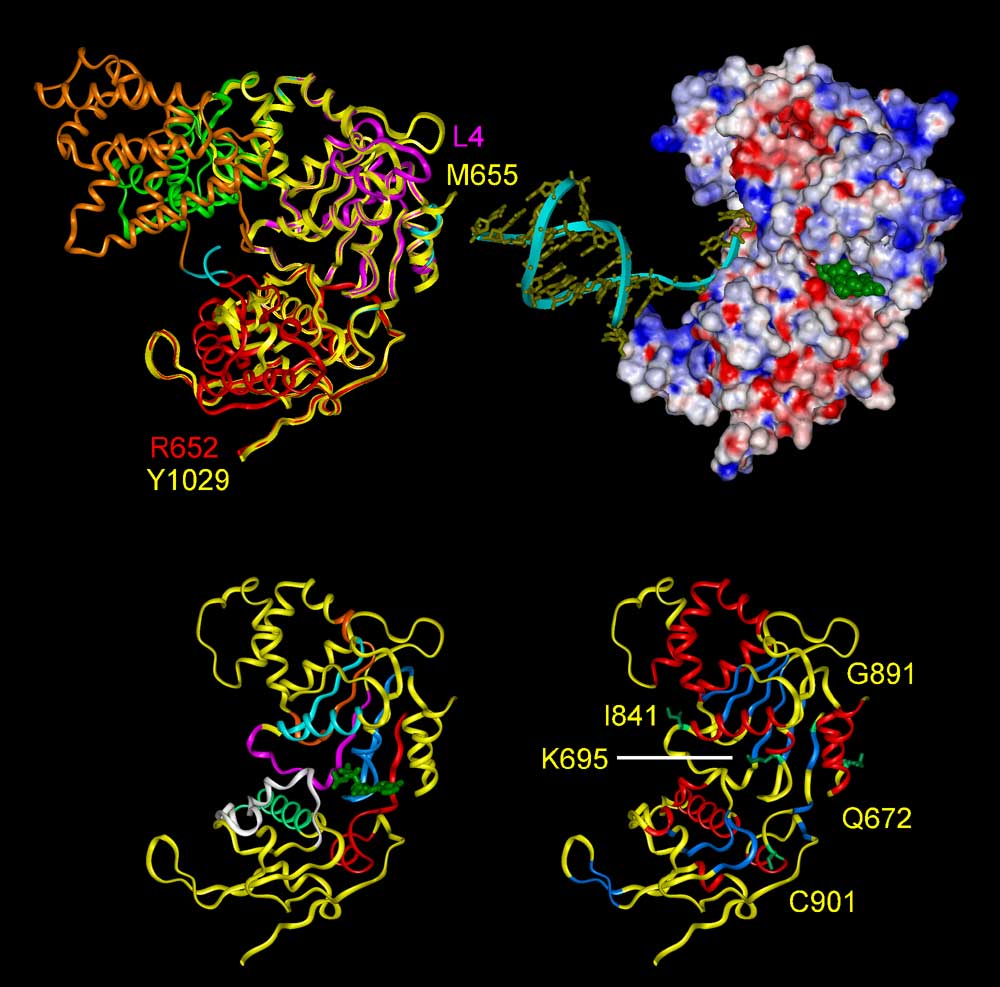

Fig. 3. (Upper left) Superposition of ribbon diagrams of the template protein
PcrA DNA helicase (3PJR) and the BLM helicase domain (yellow). The trace of
the PcrA helicase from residues 4 to 652 and the structural model of the BLM
protein from residues 655 to 1029 are shown. The PcrA DNA helicase is comprised
of 4 subdomains 1A (magenta), 1B (green), 2A (red), and 2B (orange); whereas
the BLM model only contains subdomains 1A and 2A.
(Upper right) Electrostatic surface of the model with the same view as left.
Negative potential is shown in red, positive potential in blue. The surface
potential is negative over most of the surface except for the DNA-binding cleft.
The docked ATP (green) is situated in the nucleotide-binding pocket between
subdomains 1A and 2A. The merged DNA (yellow) with ribbon is located at the
binding cleft with good complementarity.
(Bottom) Stereo diagram showing the structural model of the BLM helicase domain
with the same view as upper.
(Bottom left) The seven conserved helicase motifs, I, Ia, II, III, IV, V, and
VI, are shown in blue, light blue, orange, magenta, red, white, and green, respectively.
The docked ATP is displayed in green. The nucleotide binding pocket is contributed
by motifs I, II-IV, and VI; whereas the DNA binding cleft is formed by motifs
Ia, III, and V.
(Bottom right) Secondary structure elements are indicated in red for a-helices
and blue for b-strands. The residues of BS-causing mutations are displayed with
sidechains in green and residue K695 corresponding to mutation K703A on mouse
BLM is also displayed.